How to burn carbon dioxide? Carbon dioxide liquid (CO2, carbon dioxide, carbon dioxide) Carbon dioxide burns or not.
We all know from school that carbon dioxide is emitted into the atmosphere as a product of human and animal life, that is, it is what we exhale. In fairly small quantities, it is absorbed by plants and converted into oxygen. One of the causes of global warming is carbon dioxide, or in other words carbon dioxide.
But not everything is as bad as it seems at first glance, because humanity has learned to use it in a wide area of its activity for good purposes. For example, carbon dioxide is used in carbonated waters, or in the food industry it can be found on the label under code E290 as a preservative. Quite often, carbon dioxide acts as a leavening agent in flour products, where it enters during the preparation of dough. Most often, carbon dioxide is stored in a liquid state in special cylinders, which are used repeatedly and can be refilled. You can find out more about this on the website https://wice24.ru/product/uglekislota-co2. It can be found both in a gaseous state and in the form of dry ice, but storing it in a liquefied state is much more profitable.
Biochemists have proven that fertilizing the air with carbon gas is a very good means of obtaining large yields from various crops. This theory has long found its practical application. Thus, in Holland, flower growers effectively use carbon dioxide to fertilize various flowers (gerberas, tulips, roses) in greenhouse conditions. And if previously the necessary climate was created by burning natural gas (this technology was considered ineffective and harmful to the environment), today carbon gas reaches the plants through special tubes with holes and is used in the required quantity, mainly in winter.
Carbon dioxide is also widely used in the fire industry as a fire extinguisher refill. Carbon dioxide in cans has found its way into air guns, and in aircraft modeling it serves as a source of energy for engines.
In its solid state, CO2 has, as already mentioned, the name dry ice, and is used in the food industry for food storage. It is worth noting that compared to ordinary ice, dry ice has a number of advantages, including high cooling capacity (2 times higher than usual), and when it evaporates, no by-products remain.
And these are not all the areas where carbon dioxide is used effectively and efficiently.
Key words: Where is carbon dioxide used, Use of carbon dioxide, industry, in everyday life, refilling cylinders, carbon dioxide storage, E290
Carbon dioxide, carbon monoxide, carbon dioxide - all these are names for one substance known to us as carbon dioxide. So what properties does this gas have, and what are its areas of application?
Carbon dioxide and its physical properties
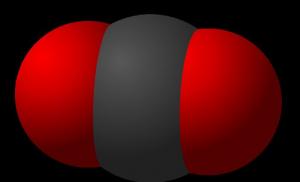
Carbon dioxide consists of carbon and oxygen. The formula for carbon dioxide looks like this – CO₂. In nature, it is formed during the combustion or decay of organic substances. The gas content in the air and mineral springs is also quite high. In addition, humans and animals also emit carbon dioxide when they exhale.

Rice. 1. Carbon dioxide molecule.
Carbon dioxide is a completely colorless gas and cannot be seen. It also has no smell. However, with high concentrations, a person may develop hypercapnia, that is, suffocation. Lack of carbon dioxide can also cause health problems. As a result of a lack of this gas, the opposite condition to suffocation can develop - hypocapnia.
If you place carbon dioxide in low temperature conditions, then at -72 degrees it crystallizes and becomes like snow. Therefore, carbon dioxide in a solid state is called “dry snow.”

Rice. 2. Dry snow – carbon dioxide.
Carbon dioxide is 1.5 times denser than air. Its density is 1.98 kg/m³. The chemical bond in the carbon dioxide molecule is covalent polar. It is polar due to the fact that oxygen has a higher electronegativity value.
An important concept in the study of substances is molecular and molar mass. The molar mass of carbon dioxide is 44. This number is formed from the sum of the relative atomic masses of the atoms that make up the molecule. The values of relative atomic masses are taken from the table of D.I. Mendeleev and are rounded to whole numbers. Accordingly, the molar mass of CO₂ = 12+2*16.
To calculate the mass fractions of elements in carbon dioxide, it is necessary to follow the formula for calculating the mass fractions of each chemical element in a substance.
n– number of atoms or molecules.
A r– relative atomic mass of a chemical element.
Mr– relative molecular mass of the substance.
Let's calculate the relative molecular mass of carbon dioxide.
Mr(CO₂) = 14 + 16 * 2 = 44 w(C) = 1 * 12 / 44 = 0.27 or 27% Since the formula of carbon dioxide includes two oxygen atoms, then n = 2 w(O) = 2 * 16 / 44 = 0.73 or 73%
Answer: w(C) = 0.27 or 27%; w(O) = 0.73 or 73%
Chemical and biological properties of carbon dioxide
Carbon dioxide has acidic properties because it is an acidic oxide, and when dissolved in water it forms carbonic acid:
CO₂+H₂O=H₂CO₃
Reacts with alkalis, resulting in the formation of carbonates and bicarbonates. This gas does not burn. Only certain active metals, such as magnesium, burn in it.
When heated, carbon dioxide breaks down into carbon monoxide and oxygen:
2CO₃=2CO+O₃.
Like other acidic oxides, this gas easily reacts with other oxides:
СaO+Co₃=CaCO₃.
Carbon dioxide is part of all organic substances. The circulation of this gas in nature is carried out with the help of producers, consumers and decomposers. In the process of life, a person produces approximately 1 kg of carbon dioxide per day. When we inhale, we receive oxygen, but at this moment carbon dioxide is formed in the alveoli. At this moment, an exchange occurs: oxygen enters the blood, and carbon dioxide comes out.
Carbon dioxide is produced during the production of alcohol. This gas is also a by-product in the production of nitrogen, oxygen and argon. The use of carbon dioxide is necessary in the food industry, where carbon dioxide acts as a preservative, and carbon dioxide in liquid form is found in fire extinguishers.
The most common processes for the formation of this compound are the rotting of animal and plant remains, the combustion of various types of fuel, and the respiration of animals and plants. For example, one person emits about a kilogram of carbon dioxide into the atmosphere per day. Carbon monoxide and dioxide can also be formed in inanimate nature. Carbon dioxide is released during volcanic activity and can also be produced from mineral water sources. Carbon dioxide is found in small quantities in the Earth's atmosphere.
The peculiarities of the chemical structure of this compound allow it to participate in many chemical reactions, the basis for which is carbon dioxide.
Formula
In the compound of this substance, the tetravalent carbon atom forms a linear bond with two oxygen molecules. The appearance of such a molecule can be represented as follows:

The hybridization theory explains the structure of the carbon dioxide molecule as follows: the two existing sigma bonds are formed between the sp orbitals of the carbon atoms and the two 2p orbitals of oxygen; The p-orbitals of carbon, which do not take part in hybridization, are bonded in conjunction with similar orbitals of oxygen. In chemical reactions, carbon dioxide is written as: CO 2.
Physical properties
Under normal conditions, carbon dioxide is a colorless, odorless gas. It is heavier than air, which is why carbon dioxide can behave like a liquid. For example, it can be poured from one container to another. This substance is slightly soluble in water - about 0.88 liters of CO 2 dissolve in one liter of water at 20 ⁰C. A slight decrease in temperature radically changes the situation - 1.7 liters of CO 2 can dissolve in the same liter of water at 17⁰C. With strong cooling, this substance precipitates in the form of snow flakes - the so-called “dry ice” is formed. This name comes from the fact that at normal pressure the substance, bypassing the liquid phase, immediately turns into a gas. Liquid carbon dioxide is formed at a pressure just above 0.6 MPa and at room temperature.

Chemical properties
When interacting with strong oxidizing agents, 4-carbon dioxide exhibits oxidizing properties. The typical reaction of this interaction is:
C + CO 2 = 2CO.
Thus, with the help of coal, carbon dioxide is reduced to its divalent modification - carbon monoxide.
Under normal conditions, carbon dioxide is inert. But some active metals can burn in it, removing oxygen from the compound and releasing carbon gas. A typical reaction is the combustion of magnesium:
2Mg + CO 2 = 2MgO + C.
During the reaction, magnesium oxide and free carbon are formed.

In chemical compounds, CO 2 often exhibits the properties of a typical acid oxide. For example, it reacts with bases and basic oxides. The result of the reaction is carbonic acid salts.
For example, the reaction of a compound of sodium oxide with carbon dioxide can be represented as follows:
Na 2 O + CO 2 = Na 2 CO 3;
2NaOH + CO 2 = Na 2 CO 3 + H 2 O;
NaOH + CO 2 = NaHCO 3.
Carbonic acid and CO 2 solution
Carbon dioxide in water forms a solution with a small degree of dissociation. This solution of carbon dioxide is called carbonic acid. It is colorless, weakly expressed and has a sour taste.
Recording a chemical reaction:
CO 2 + H 2 O ↔ H 2 CO 3.
The equilibrium is shifted quite strongly to the left - only about 1% of the initial carbon dioxide is converted into carbonic acid. The higher the temperature, the fewer carbonic acid molecules in the solution. When the compound boils, it disappears completely, and the solution disintegrates into carbon dioxide and water. The structural formula of carbonic acid is presented below.

Properties of carbonic acid
Carbonic acid is very weak. In solutions, it breaks down into hydrogen ions H + and compounds HCO 3 -. CO 3 - ions are formed in very small quantities.
Carbonic acid is dibasic, so the salts formed by it can be medium and acidic. In the Russian chemical tradition, medium salts are called carbonates, and strong salts are called bicarbonates.
Qualitative reaction
One possible way to detect carbon dioxide gas is to change the clarity of the lime mortar.
Ca(OH) 2 + CO 2 = CaCO 3 ↓ + H 2 O.
This experience is known from a school chemistry course. At the beginning of the reaction, a small amount of white precipitate is formed, which subsequently disappears when carbon dioxide is passed through water. The change in transparency occurs because during the interaction process, an insoluble compound - calcium carbonate - is converted into a soluble substance - calcium bicarbonate. The reaction proceeds along this path:
CaCO 3 + H 2 O + CO 2 = Ca(HCO 3) 2.
Production of carbon dioxide
If you need to get a small amount of CO2, you can start the reaction of hydrochloric acid with calcium carbonate (marble). The chemical notation for this interaction looks like this:
CaCO 3 + HCl = CaCl 2 + H 2 O + CO 2.
Also for this purpose, combustion reactions of carbon-containing substances, for example acetylene, are used:
CH 4 + 2O 2 → 2H 2 O + CO 2 -.
A Kipp apparatus is used to collect and store the resulting gaseous substance.
For the needs of industry and agriculture, the scale of carbon dioxide production must be large. A popular method for this large-scale reaction is to burn limestone, which produces carbon dioxide. The reaction formula is given below:
CaCO 3 = CaO + CO 2.
Applications of carbon dioxide
The food industry, after large-scale production of “dry ice,” switched to a fundamentally new method of storing food. It is indispensable in the production of carbonated drinks and mineral water. The CO 2 content in drinks gives them freshness and significantly increases their shelf life. And carbidization of mineral waters allows you to avoid mustiness and unpleasant taste.

In cooking, the method of extinguishing citric acid with vinegar is often used. The carbon dioxide released gives fluffiness and lightness to confectionery products.
This compound is often used as a food additive to increase the shelf life of food products. According to international standards for the classification of chemical additives in products, they are coded E 290,
Powdered carbon dioxide is one of the most popular substances included in fire extinguishing mixtures. This substance is also found in fire extinguisher foam.
It is best to transport and store carbon dioxide in metal cylinders. At temperatures above 31⁰C, the pressure in the cylinder can reach critical and liquid CO 2 will go into a supercritical state with a sharp rise in operating pressure to 7.35 MPa. The metal cylinder can withstand internal pressure up to 22 MPa, so the pressure range at temperatures above thirty degrees is considered safe.
, carbon dioxide, properties of carbon dioxide, production of carbon dioxide
It is not suitable for supporting life. However, it is this that plants “feed” on, turning it into organic substances. In addition, it is a kind of “blanket” for the Earth. If this gas suddenly disappeared from the atmosphere, the Earth would become much cooler and rain would virtually disappear.
"Blanket of the Earth"
(carbon dioxide, carbon dioxide, CO 2) is formed when two elements combine: carbon and oxygen. It is formed during the combustion of coal or hydrocarbon compounds, during the fermentation of liquids, and also as a product of the respiration of people and animals. It is also found in small quantities in the atmosphere, from where it is assimilated by plants, which, in turn, produce oxygen.
Carbon dioxide is colorless and heavier than air. Freezes at −78.5°C to form snow consisting of carbon dioxide. In aqueous solution it forms carbonic acid, but it is not stable enough to be easily isolated.
Carbon dioxide is the Earth's blanket. It easily transmits ultraviolet rays that heat our planet and reflects infrared rays emitted from its surface into outer space. And if carbon dioxide suddenly disappears from the atmosphere, this will primarily affect the climate. It will become much cooler on Earth, and rain will fall very rarely. It’s not hard to guess where this will ultimately lead.
True, such a catastrophe does not yet threaten us. Quite the contrary. The combustion of organic substances: oil, coal, natural gas, wood - gradually increases the carbon dioxide content in the atmosphere. This means that over time we must expect significant warming and humidification of the earth’s climate. By the way, old-timers believe that it is already noticeably warmer than it was in the days of their youth...
Carbon dioxide is released liquid low temperature, high pressure liquid And gaseous. It is obtained from waste gases from ammonia and alcohol production, as well as from special fuel combustion and other industries. Gaseous carbon dioxide is a colorless and odorless gas at a temperature of 20 ° C and a pressure of 101.3 kPa (760 mm Hg), density - 1.839 kg / m 3. Liquid carbon dioxide is simply a colorless, odorless liquid.
Non-toxic and non-explosive. At concentrations of more than 5% (92 g/m3), carbon dioxide has a harmful effect on human health - it is heavier than air and can accumulate in poorly ventilated areas near the floor. This reduces the volume fraction of oxygen in the air, which can cause oxygen deficiency and suffocation.
Producing carbon dioxide
In industry, carbon dioxide is obtained from furnace gases, from decomposition products of natural carbonates(limestone, dolomite). The mixture of gases is washed with a solution of potassium carbonate, which absorbs carbon dioxide, turning into bicarbonate. When heated, the bicarbonate solution decomposes, releasing carbon dioxide. During industrial production, gas is pumped into cylinders.
In laboratory conditions small amounts are obtained interaction of carbonates and bicarbonates with acids, for example, marble with hydrochloric acid.
"Dry ice" and other beneficial properties of carbon dioxide
Carbon dioxide is used quite widely in everyday practice. For example, sparkling water with the addition of aromatic essences - a wonderful refreshing drink. IN food industry carbon dioxide is also used as a preservative - it is indicated on the packaging under the code E290, and also as a dough leavening agent.
Carbon dioxide fire extinguishers used in fires. Biochemists have found that fertilization... of the air with carbon dioxide a very effective means for increasing the yield of various crops. Perhaps this fertilizer has a single, but significant drawback: it can only be used in greenhouses. At plants that produce carbon dioxide, liquefied gas is packaged in steel cylinders and sent to consumers. If you open the valve, snow comes out with a hiss. What kind of miracle?
Everything is explained simply. The work expended on compressing the gas is significantly less than that required to expand it. And in order to somehow compensate for the resulting deficit, carbon dioxide cools sharply, turning into "dry ice". It is widely used to preserve food and has significant advantages over ordinary ice: firstly, its “cooling capacity” is twice as high per unit weight; secondly, it evaporates without a trace.
Carbon dioxide is used as an active medium in wire welding, since at arc temperature, carbon dioxide decomposes into carbon monoxide CO and oxygen, which, in turn, interacts with the liquid metal, oxidizing it.
Carbon dioxide in cans is used in air guns and as energy source for engines in aircraft modeling.
Carbon dioxide is a colorless gas with a barely perceptible odor, non-toxic, heavier than air. Carbon dioxide is widely distributed in nature. It dissolves in water, forming carbonic acid H 2 CO 3, giving it a sour taste. The air contains about 0.03% carbon dioxide. The density is 1.524 times greater than the density of air and is equal to 0.001976 g/cm 3 (at zero temperature and pressure 101.3 kPa). Ionization potential 14.3V. Chemical formula - CO 2.
In welding production the term is used "carbon dioxide" cm. . In the “Rules for the Design and Safe Operation of Pressure Vessels” the term "carbon dioxide", and in - term "carbon dioxide".
There are many ways to produce carbon dioxide, the main ones are discussed in the article.
The density of carbon dioxide depends on pressure, temperature and the state of aggregation in which it is found. At atmospheric pressure and a temperature of -78.5°C, carbon dioxide, bypassing the liquid state, turns into a white snow-like mass "dry ice".
Under a pressure of 528 kPa and at a temperature of -56.6 ° C, carbon dioxide can be in all three states (the so-called triple point).
Carbon dioxide is thermally stable, dissociating into carbon monoxide only at temperatures above 2000°C.
Carbon dioxide is first gas to be described as a discrete substance. In the seventeenth century, a Flemish chemist Jan Baptist van Helmont (Jan Baptist van Helmont) noticed that after burning coal in a closed vessel, the mass of ash was much less than the mass of the burned coal. He explained this by saying that coal was transformed into an invisible mass, which he called “gas.”
The properties of carbon dioxide were studied much later in 1750. Scottish physicist Joseph Black (Joseph Black).
He discovered that limestone (calcium carbonate CaCO 3), when heated or reacted with acids, releases a gas, which he called “bound air.” It turned out that “bound air” is denser than air and does not support combustion.
CaCO 3 + 2HCl = CO 2 + CaCl 2 + H 2 O
By passing “bound air” i.e. carbon dioxide CO 2 through an aqueous solution of lime Ca(OH) 2 calcium carbonate CaCO 3 is deposited to the bottom. Joseph Black used this experiment to prove that carbon dioxide is released through animal respiration.
CaO + H 2 O = Ca(OH) 2
Ca(OH) 2 + CO 2 = CaCO 3 + H 2 O
Liquid carbon dioxide is a colorless, odorless liquid whose density varies greatly with temperature. It exists at room temperature only at pressures above 5.85 MPa. The density of liquid carbon dioxide is 0.771 g/cm 3 (20°C). At temperatures below +11°C it is heavier than water, and above +11°C it is lighter.
The specific gravity of liquid carbon dioxide varies significantly with temperature, therefore, the amount of carbon dioxide is determined and sold by weight. The solubility of water in liquid carbon dioxide in the temperature range 5.8-22.9°C is not more than 0.05%.
Liquid carbon dioxide turns into gas when heat is supplied to it. Under normal conditions (20°C and 101.3 kPa) When 1 kg of liquid carbon dioxide evaporates, 509 liters of carbon dioxide are formed. When gas is withdrawn too quickly, the pressure in the cylinder decreases and the heat supply is insufficient, the carbon dioxide cools, the rate of its evaporation decreases and when it reaches the “triple point” it turns into dry ice, which clogs the hole in the reduction gear, and further gas extraction stops. When heated, dry ice directly turns into carbon dioxide, bypassing the liquid state. To evaporate dry ice, it is necessary to supply significantly more heat than to evaporate liquid carbon dioxide - therefore, if dry ice has formed in the cylinder, it evaporates slowly.
Liquid carbon dioxide was first produced in 1823. Humphry Davy(Humphry Davy) and Michael Faraday(Michael Faraday).
Solid carbon dioxide "dry ice" resembles snow and ice in appearance. The carbon dioxide content obtained from dry ice briquettes is high - 99.93-99.99%. Moisture content is in the range of 0.06-0.13%. Dry ice, being in the open air, evaporates rapidly, so containers are used for its storage and transportation. Carbon dioxide is produced from dry ice in special evaporators. Solid carbon dioxide (dry ice), supplied in accordance with GOST 12162.
Carbon dioxide is most often used:
- to create a protective environment for metals;
- in the production of carbonated drinks;
- refrigeration, freezing and storage of food products;
- for fire extinguishing systems;
- for cleaning surfaces with dry ice.
The density of carbon dioxide is quite high, which allows the arc reaction space to be protected from contact with air gases and prevents nitriding at relatively low carbon dioxide consumption in the jet. Carbon dioxide is, during the welding process, it interacts with the weld metal and has an oxidizing and also carburizing effect on the metal of the weld pool.
Previously obstacles to the use of carbon dioxide as a protective medium were in the seams. The pores were caused by boiling of the solidifying metal of the weld pool from the release of carbon monoxide (CO) due to its insufficient deoxidation.
At high temperatures, carbon dioxide dissociates to form highly active free, monoatomic oxygen:
Oxidation of the weld metal released free from carbon dioxide during welding is neutralized by the content of an additional amount of alloying elements with a high affinity for oxygen, most often silicon and manganese (in excess of the amount required for alloying the weld metal) or fluxes introduced into the welding zone (welding).
Both carbon dioxide and carbon monoxide are practically insoluble in solid and molten metal. The free active oxidizes the elements present in the weld pool depending on their oxygen affinity and concentration according to the equation:
Me + O = MeO
where Me is a metal (manganese, aluminum, etc.).
In addition, carbon dioxide itself reacts with these elements.
As a result of these reactions, when welding in carbon dioxide, significant burnout of aluminum, titanium and zirconium is observed, and less intense burnout of silicon, manganese, chromium, vanadium, etc.
The oxidation of impurities occurs especially vigorously at . This is due to the fact that when welding with a consumable electrode, the interaction of the molten metal with the gas occurs when a drop remains at the end of the electrode and in the weld pool, and when welding with a non-consumable electrode, it occurs only in the pool. As is known, the interaction of gas with metal in the arc gap occurs much more intensely due to the high temperature and larger contact surface of the metal with the gas.
Due to the chemical activity of carbon dioxide in relation to tungsten, welding in this gas is carried out only with a consumable electrode.
Carbon dioxide is non-toxic and non-explosive. At concentrations of more than 5% (92 g/m3), carbon dioxide has a harmful effect on human health, since it is heavier than air and can accumulate in poorly ventilated areas near the floor. This reduces the volume fraction of oxygen in the air, which can cause oxygen deficiency and suffocation. Premises where welding is carried out using carbon dioxide must be equipped with general supply and exhaust ventilation. The maximum permissible concentration of carbon dioxide in the air of the working area is 9.2 g/m 3 (0.5%).
Carbon dioxide is supplied by . To obtain high-quality seams, gaseous and liquefied carbon dioxide of the highest and first grades is used.
Carbon dioxide is transported and stored in steel cylinders or large-capacity tanks in a liquid state, followed by gasification at the plant, with a centralized supply to welding stations through ramps. A standard one with a water capacity of 40 liters is filled with 25 kg of liquid carbon dioxide, which at normal pressure occupies 67.5% of the volume of the cylinder and produces 12.5 m 3 of carbon dioxide upon evaporation. Air accumulates in the upper part of the cylinder along with carbon dioxide gas. Water, which is heavier than liquid carbon dioxide, collects at the bottom of the cylinder.
To reduce the humidity of carbon dioxide, it is recommended to install the cylinder with the valve down and, after settling for 10...15 minutes, carefully open the valve and release moisture from the cylinder. Before welding, it is necessary to release a small amount of gas from a normally installed cylinder to remove any air trapped in the cylinder. Some of the moisture is retained in carbon dioxide in the form of water vapor, worsening the welding of the seam.
When gas is released from the cylinder, due to the throttling effect and heat absorption during the evaporation of liquid carbon dioxide, the gas cools significantly. With intensive gas extraction, the reducer may become clogged with frozen moisture contained in carbon dioxide, as well as dry ice. To avoid this, when extracting carbon dioxide, a gas heater is installed in front of the reducer. The final removal of moisture after the gearbox is carried out with a special desiccant filled with glass wool and calcium chloride, silica gel, copper sulfate or other moisture absorbers
The carbon dioxide cylinder is painted black, with the words “CARBON ACID” written in yellow letters..